Striate+™ collagen membrane
Natural collagen membrane with bilayer structure for GBR and GTR

Striate+™ is a porcine-derived resorbable collagen barrier membrane for guided bone and tissue regeneration. It is the next generation of collagen membranes which, due to its unique manufacturing process, creates a favorable environment for rapid regeneration of high-quality bone and soft tissue. The membrane can be used before or simultaneously with implantation as well as with sinus floor elevation techniques. Due to the properties of the bilayer structure, it can also be applied in the surgical treatment of periodontal defects.
Product features
- Preservation of the collagen architecture of the original tissue due to the optimized manufacturing process. This results in very good processing properties and a natural degradation profile.
- Easy fixation – the membrane can be sutured, screw-retained or pinned without tearing.
- Minimized inflammatory response
- Predictable resorption within four to six months
- Optimal wound stabilization and healing
- Ideal soft tissue integration yielding aesthetic results
The SMRT™ manufacturing process¹
- The Scaffold Matrix Regenerative Therapy (SMRT™) manufacturing process removes:
- immunogenic impurities, including alpha-gal
- GAGs, DNA and lipids without damaging the native collagen structure
- The Striate+ bioactive elements promote cell ingrowth and adhesion.
- The network of collagen fibers provides an ideal microstructure for cell proliferation and attachment.
Successful bone regeneration¹
Rapid and predictable bone regeneration observed during implant placement in the two-stage therapeutic procedure after guided bone regeneration with Striate+.
- Early vertical bone regeneration was observed at 12 days post-treatment.
- Rapid gain in bone hight maintained during healing.
- Vertical bone regeneration stabilizes implant, improves long-term survival and esthetic outcomes.
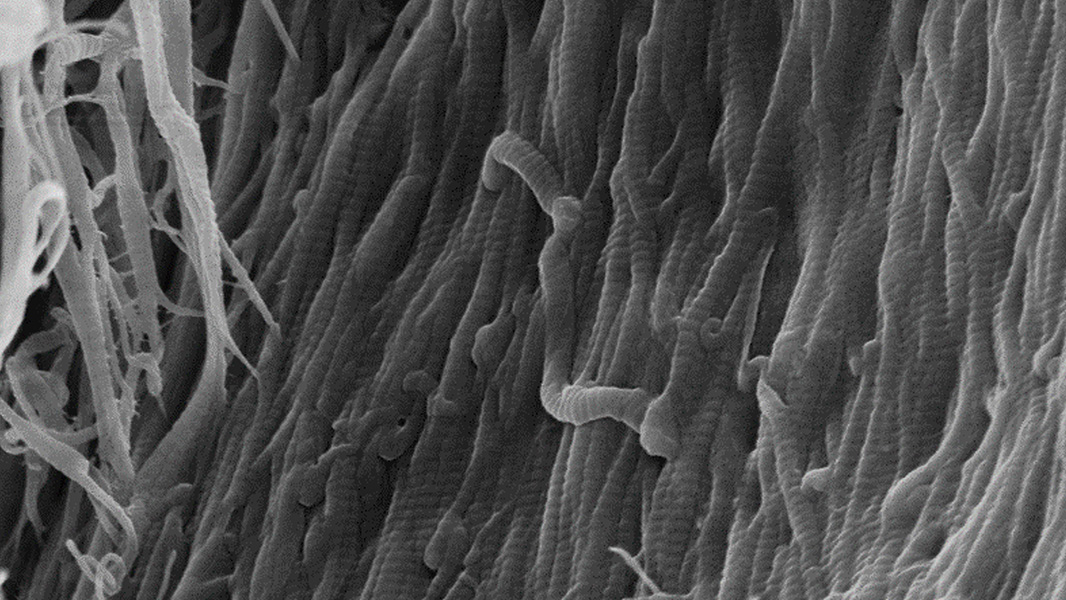
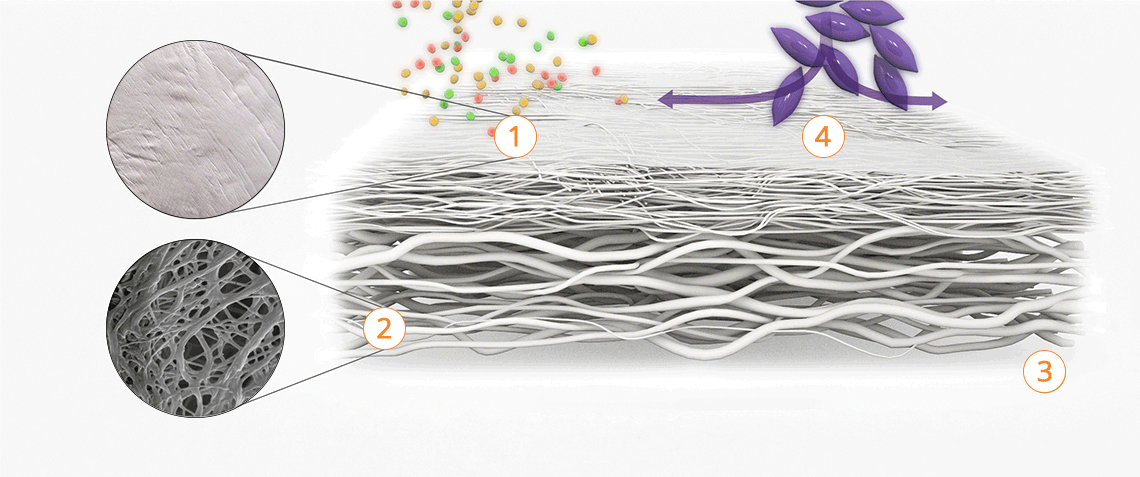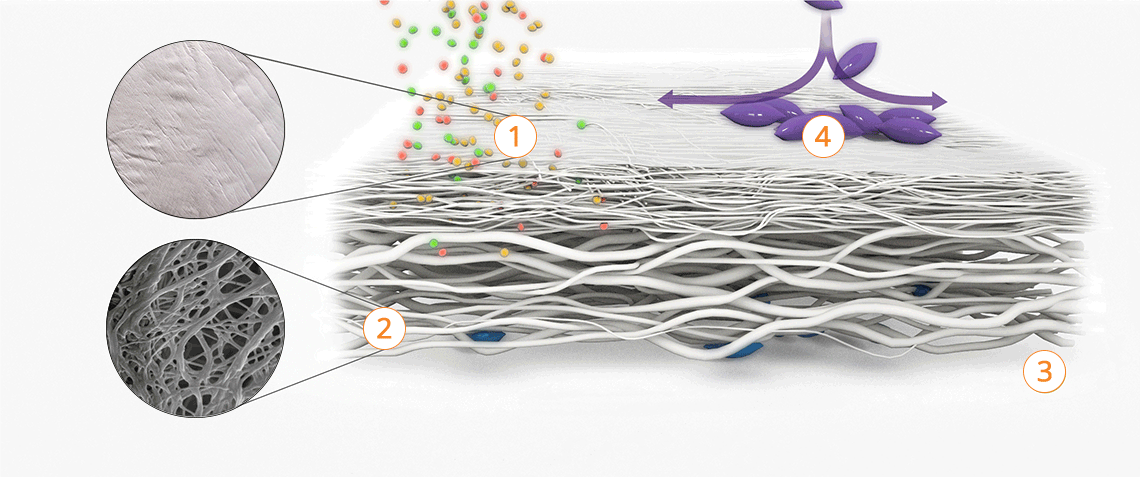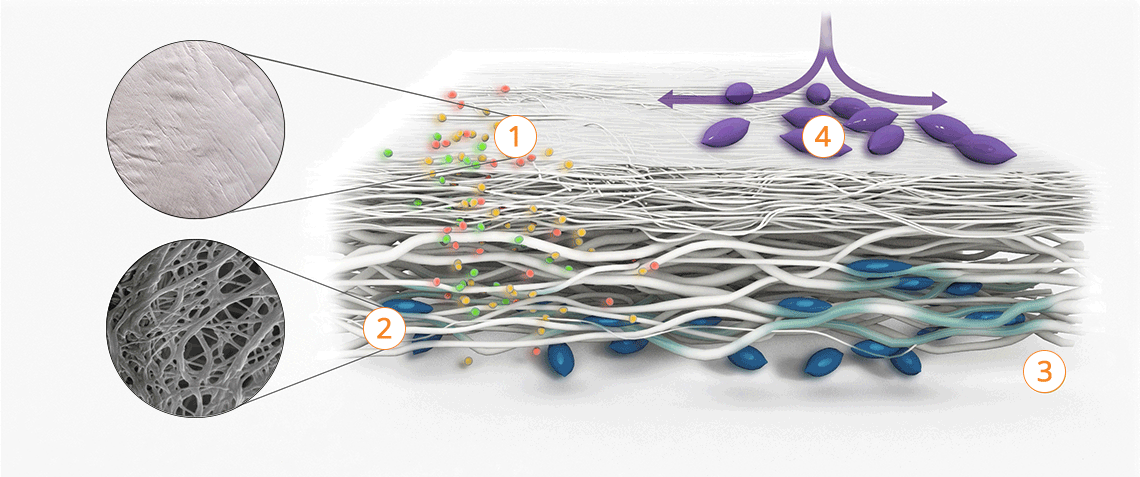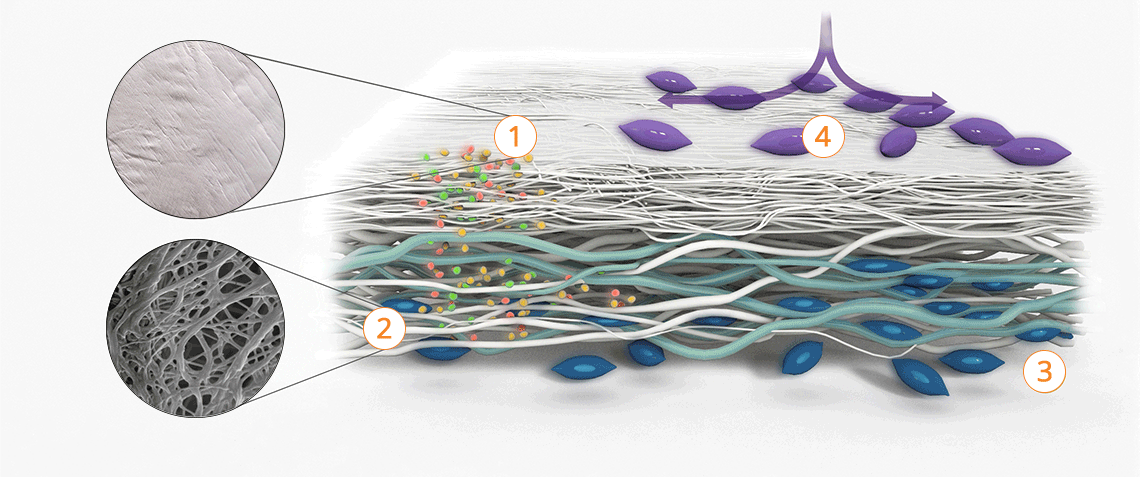
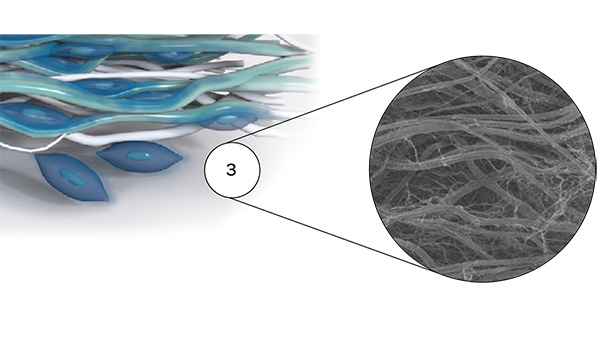
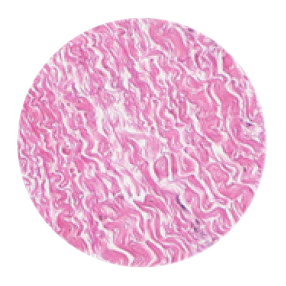
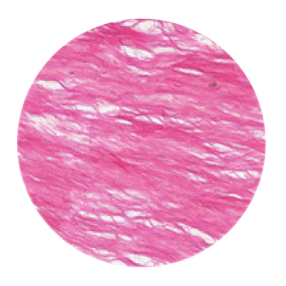
The tissue structure of Striate+
Improved bone regeneration
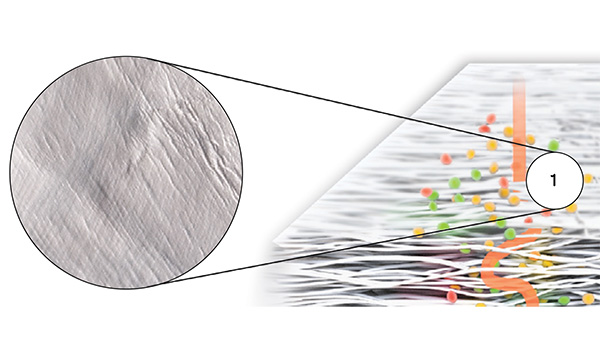
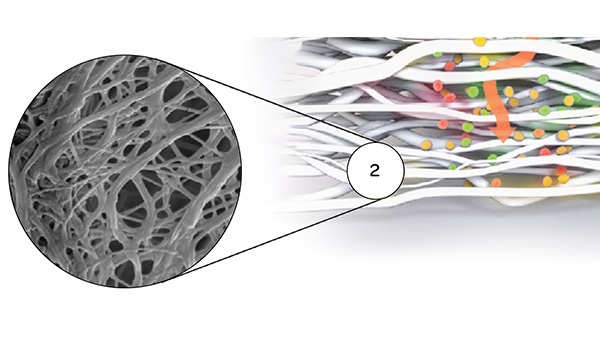
Striate+ collagen membranes feature a bilayer structure with a rough side and a smooth side to stimulate specific biological responses. The rough side, which faces the bone defect, consists of a loose distribution of collagen bundles that forms an open scaffold for the entry of osteogenic cells. The smooth side facing the soft tissue consists of densely packed collagen bundles arranged in parallel, which in their barrier function prevent the migration of the gingival cells into the defect.
Striate+ was developed to protect the bone graft from epithelial cell ingrowth and to create a favorable environment for osteogenesis – with a predictable resorption time for protected bone regeneration.
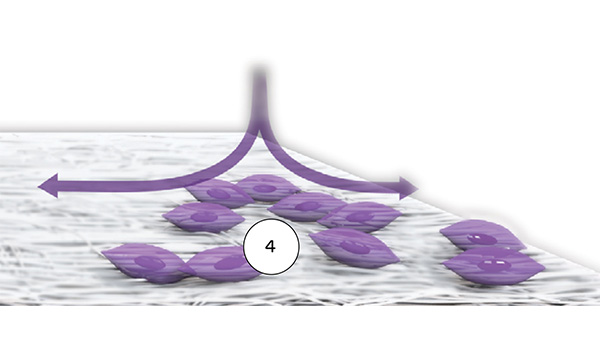
Properties of the different surface structures
Striate+ has a bilayered structure with two functional sides:
- The smooth side acts as a barrier against the in-growth of gingival and epithelial cells.
- The rough side is made of a porous network of collagen fibers that promote osteogenesis, referred to as "Bioactive Chamber".
Advantages when using Striate+
Easy handling and shorter treatment times
The Striate+ collagen membrane is mostly used in combination with bone substitute material or the patient's own bone. It is an effective barrier to allow bone regeneration in the space of the defect. The rough surface acts as an optimal guide to generate a stable bone volume of better quality2.
Striate+ is fully resorbed after approximately 26 weeks. Among other things, Striate+ is characterized by high tensile strength and tear resistance as well as adaptability during handling1.
- Non-cross-linked acellular type I collagen
Does not cause any abnormal inflammatory response1
- Easy handling
Adapts easily to the bone surface, does not collapse when hydrated
- Bilayer membrane structure
Two different surface structures that are visually distinguishable
- Dense barrier layer
Prevents the infiltration of gingival cells while allowing penetration of bioactive molecules and proteins1.
- Bioactive compartments
Enable early integration of bone-forming cells and provide a favorable environment for osteogenesis1.
Orthocell Ltd. – over 15 years of experience
Innovative products for regeneration therapy
Orthocell Ltd. is a leading Australian biotechnology company whose mission is to translate world-class research into commercially viable and life-changing products. The company specializes in regenerative medicine and is dedicated to the development of breakthrough products for the treatment of musculoskeletal disorders. Orthocell Ltd. has established a quality-controlled manufacturing facility at its headquarters in Perth, Australia. The facility is licensed by the Therapeutic Goods Administration (TGA) for the production of human tendon cells (tenocytes) and cartilage cells (chondrocytes) for the regeneration of damaged tendons and cartilage. The manufacturing facility is also approved according to ISO 13485 for the production of the CelGro™ collagen membrane. Under license from BioHorizons Inc., this membrane is marketed worldwide under the name Striate+.*
The SMRT manufacturing process¹
Orthocell Ltd's SMRT manufacturing process removes porcine DNA and cellular components, resulting in a highly purified, bio-compatible Type I collagen membrane. The complete removal of immunogenic impurities as well as the galactose-alpha-1,3-galactose (α-Gal) ensures high biocompatibility and full integration into the patient tissue during the healing process.
- The absence of inflammatory or foreign body reactions promotes a favorable clinical outcome.
- Preservation of the natural collagen architecture results in a more stable membrane with improved handling properties and an optimal degradation profile.
Dr. Gil Alcoforado, Lisbon, Portugal
The bilayer structure which supports cellular ingrowth as well as resorption profile leads to optimal tissue integration and wound stabilization. Striate+ promotes an optimal and predictable result, even in difficult cases.
Dr. Rémy Tanimura, Paris, France
Thanks to optimal wound stabilization, both bone and soft tissue regeneration in this case of vestibular contour correction are promoted facilitating tissue healing, integration and regeneration (2 years follow- up). Soft tissue healing exhibits less dehiscence and higher therapeutic safety when using Striate+. The membrane can be positioned over the bone and the dental implants – ideally without any shifting.
Dr. Brent Allan, Perth, Australia
The use of the Striate+ membrane sets the foundation for predictable results. Due to its therapeutic safety, it forms the basis for long-term stable implant treatment. High-quality, mature bone was regenerated at all implant sites – both in vertical and horizontal dimensions.
References
[1] Allan B. et al. Collagen Membrane for Guided Bone Regeneration in Dental and Orthopedic Applications. Tissue Engineering 2020.
[2] Data on file, Orthocell Ltd. Striate+™ – Mechanical testing, Preclinical Data.
[3] Tai et al. Systematic evaluation of three porcine-derived collagen membranes for guided bone regeneration. Biomater Transl. 2023. 28;4(1):41-50.
* Striate+™ is approved for sale in the European Union.
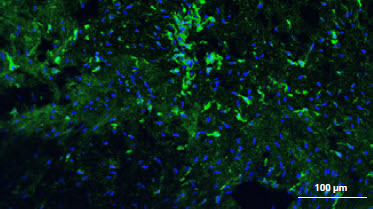
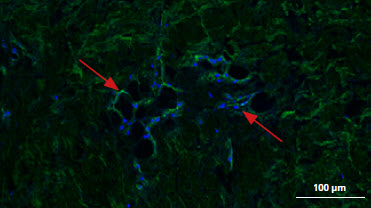
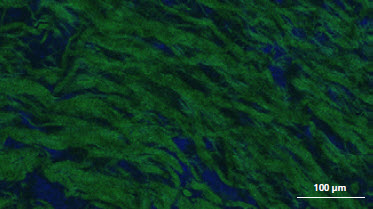
** The presence of residual cells and galactose-alpha-1,3-galactose (α-Gal) on the membranes was investigated by confocal laser scanning microscopy CLSM showing comparison between the unprocessed porcine peritoneum and Striate+. α-Gal is labeled with isolectin GS-IB4 (green) and the cell nucleus is labeled with DAPI (blue). CLSM images confirm that Striate+ contains no cellular components and has no detectable levels of α-Gal, evidenced by no fluorescence detected on both DAPI-stained and isolectin-/GS-IB4-stained images respectively.