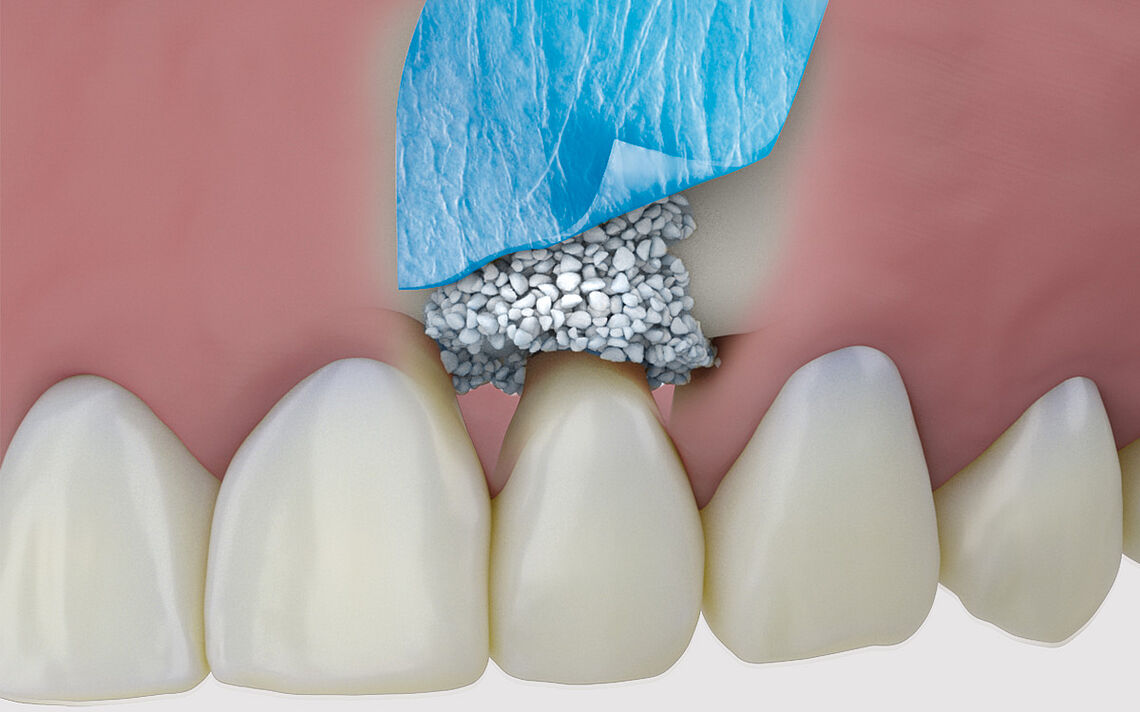
SynMax® – the synthetic alternative
Osseous integration with subsequent controlled resorption¹ ²
Besides autologous bone, bone materials of other species or synthetic materials are frequently used for the reconstruction of alveolar ridge deficiencies. While autografts are considered to be the "gold standard" in terms of biocompatibility, their harvesting - combined with a second surgical intervention - is accompanied by pain, morbidity and volume restrictions. Therefore, considerable efforts are being made to develop materials from alternative sources as well as techniques that lead to sufficient bone formation within a short period of time.
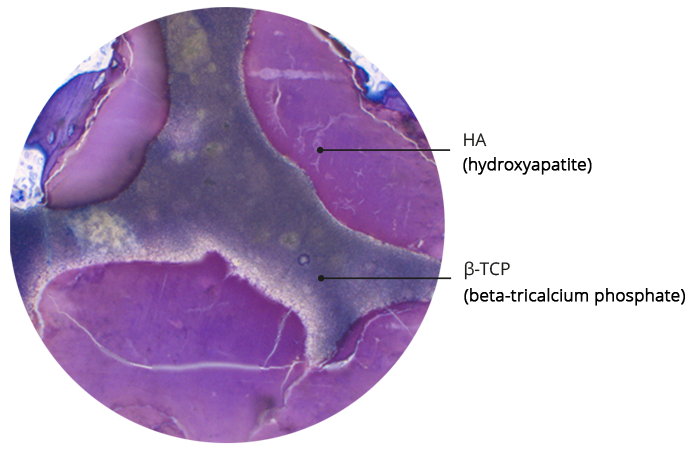
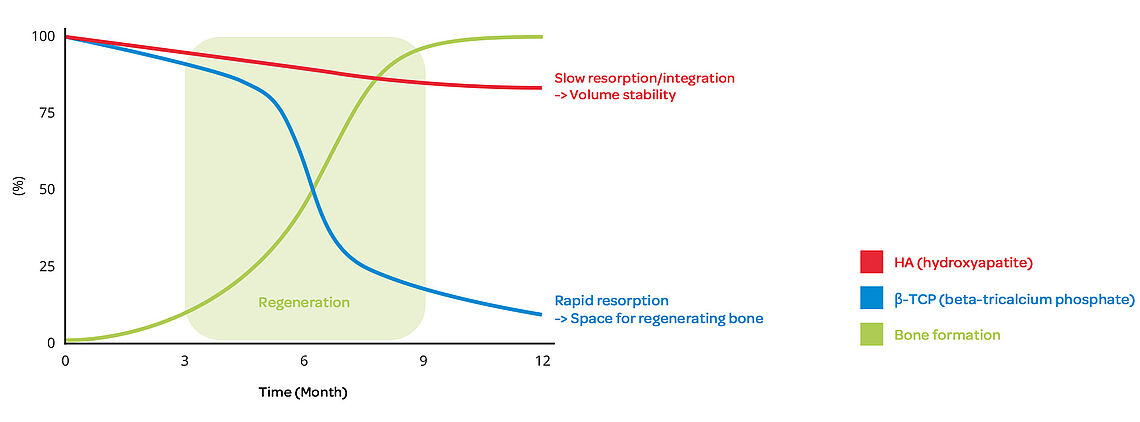
SynMax is a fully synthetic, safe and biocompatible material that, when brough into an osseous environment, serves as an osteoconductive scaffold to support the ingrowth and fusion of adjacent, vital bone. It‘s composed of 60 % hydroxyapatite and 40 % betatricalcium phosphate. After implantation the material undergoes a natural remodeling and is gradually resorbed and replaced by new bone.
Bioactive stimulation with SynMax
Due to its material properties, which enable excellent binding and release kinetics of signaling molecules and growth factors, for example, SynMax is also extremely well suited for use in combination with platelet concentrates. To accelerate regeneration, platelet-rich fibrin (e.g. L-PRF, IntraSpin®) is added, which is obtained by centrifugation from the patient's own peripheral blood. After adding, fibrin coagulates and forms a moldable substance which is easier to apply during the intraoperative filling of defects.
Bi-phasic composition of SynMax® ensures controlled resorption
SynMax acts as a temporary, osteoconductive scaffold and is gradually replaced by new bone substance as part of the natural bone remodeling process.
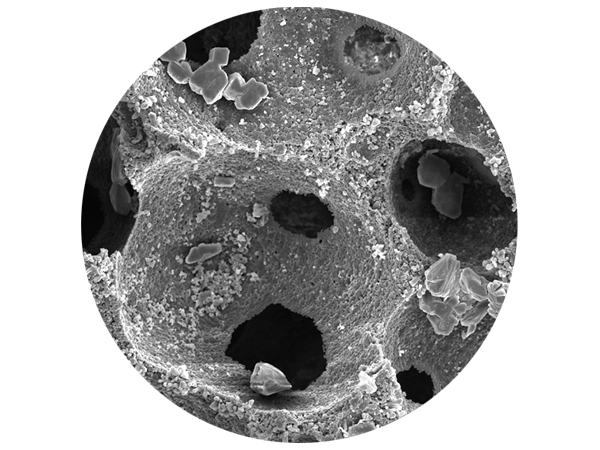
SEM analysis and histological structure of SynMax®
Physicochemical properties of suitable bone grafts
SEM analyses of SynMax demonstrate a very rough surface and a matrix of interconnected pores with a very high porosity of approx. 80 %. The interconnected pores of SynMax provide an ideal network of cavities for the ingrowth and migration of cells and blood vessels, thus promoting the formation of new vital bone.
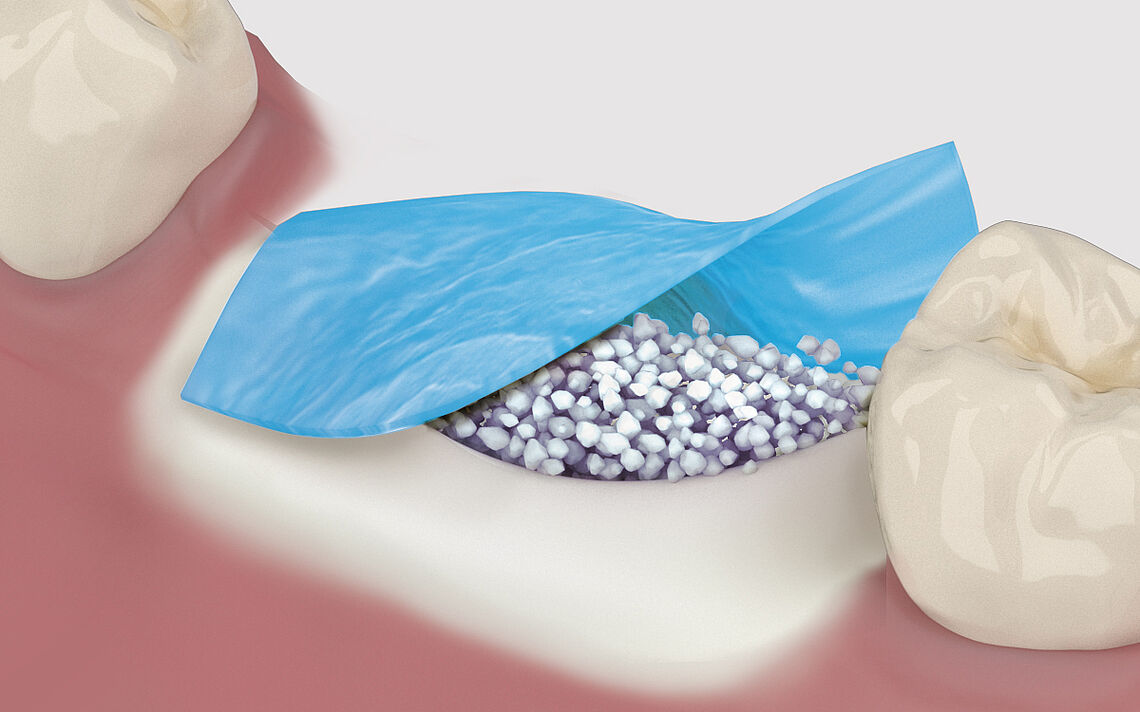
Regeneration and augmentation
The aim of any tissue regeneration technique, and bone grafting in particular, is to achieve formation of living and reactive tissue able to undergo a sustained state of remodeling to maintain the mechanical and the biologic function in the long term. If the native bone volume is insufficient for the insertion of implants, measures to augment the alveolar bone are often necessary. Augmentation can, for example, be performed after bone loss, periodontal disease, tooth extraction or trauma - depending on the indication - before or at the same time as implant placement.